EU Regulation on Cosmetic Products sets the regulatory framework for cosmetic products
This article has been translated from Finnish to English by Semantix. You can find the original article at the web adress https://www.kosmetiikka-allergia.fi/tietoa-kosmetiikasta/eun-kosmetiikka-asetus-asettaa-raamit-kosmetiikalleKirjaudu
In Finland, cosmetic products are regulated by the EU Regulation(EC) No 1223/2009 on Cosmetic Products, which came into force in its entirety on 11 July 2013. As it is a regulation, it means that it is a binding legislative act in all Member States. In addition to the EU countries, Norway, Island and Lichtenstein also follow the EU Regulation on Cosmetic Products.
The purpose of the Regulation is to strengthen the safety of cosmetic products, and the Regulation, among other things, lays down the rules for the safety assessment of the products, the allowed substances and the responsibilities of different operators to ensure the safety of the products. While the Regulation sets out the rules for the allowed composition of the products, it obliges the Member States to set a separate legislation on the surveillance of cosmetics and the language requirements. In Finland, the national Act on Cosmetic Products (492/2013) defines the competent authorities supervising cosmetic products and which labelling and product information must be presented in Finnish and Swedish.
What products are considered cosmetic products?
According to the regulation, cosmetic products may include creams, emulsions, lotions, gels and oils for the skin, face masks, tinted bases, make-up powders, after-bath powders, hygienic powders, toilet soaps, deodorant soaps, perfumes, toilet waters and eau de Cologne, bath and shower preparations, depilatories, deodorants and anti-perspirants, hair colorants, products for waving, straightening and fixing hair, hair-setting products, hair-cleansing products, hair-conditioning products, hairdressing products, shaving products, make-up and products removing make-up, products intended for application to the lips, products for care of the teeth and the mouth, products for nail care and make-up, products for external intimate hygiene, sunbathing products, products for tanning without sun, skin-whitening products and anti-wrinkle products.
Cosmetic products are intended to be applied on the external parts of the human body (or the teeth and mucous membranes of the oral cavity) with the intention to clean or perfum them, change their appearance, protect them, keep them in good condition or correct body odours. Cosmetic products are not used, for example, as a treatment to diseases. In any borderline cases, the assessment of whether a product is a cosmetic product is made on a case-by-case basis. The European Commission has published guidance documents (Borderline products) to facilitate the classification. Tattoos and false eyelashes are a good example of products that are not cosmetics.
Allowed substances
The Regulations on Cosmetic Products regulates the allowed and prohibited substances in cosmetic products, and the prohibited substances are listed in the Regulation. Also, the use of substances which are classified as carcinogenic, mutagenic or toxic to reproduction in the Regulation (EC) No 1272/2008 on classification, labelling and packaging of chemical substances (CLP legislation) are not allowed in cosmetic products. The Regulation also includes a long list of substances allowed with restrictions, including their maximum concentration, use and any warnings. The regulation also lists the allowed colorants, preservatives and UV-filters, including their allowed concentrations, in cosmetic products.
The Scientific Committee for Consumer Safety (SCCS) is responsible for the safety assessment of these substances, and the regulation of these substances is based on the evaluations made by the SCCS. Apart from the assessment of these substances, the enterprise responsible for the product is responsible for the assessment of other substances and the final product.
The European Commission maintains the CosIng databse for information on cosmetic substances and any restrictions related to them.
Labelling facilitates the correct use of products
The manufacturer of the product or the operator who places the product on the European Union market is responsible for the safety of the final product. This enterprise is responsible for ensuring that the product placed on the market has been assessed according to the Regulation and that the product complies with all the requirements of the law. The mandatory information on the cosmetic product labelling is also an important element of the product safety.
It is the consumer’s responsibility to use and store the product according to its directions, and the labelling facilitates the safe use of the products. The consumer can refer to the list of ingredients if they wish to avoid certain substances and facilitate the selection of suitable products. If the function of a cosmetic product is not apparent from its presentation, it must include directions for use. Any warnings must also be included in the packaging.
All cosmetic products must include:
|
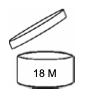
Image 1. Period-after-opening symbol of a product with maximum durability of 30 months.
Image 2. Period-after-opening symbol of a product with durability of more than 30 months.
|
If the directions of use, warnings or list of ingredients do not fit in the packaging, they must be included in a leaflet kept in the vicinity of the product. Information found elsewhere than on the packaging is indicated by a symbol of a hand pointing at a book (refer to insert symbol). Also, if the product is sold unpackaged, such as small soaps or bath balls, the required information must be presented on a leaflet made available in the immediate vicinity of the product.
 Image 3. Refer to insert symbol indicates that the directions of use, warnings and/or list of ingredients can be found in the immediate vicinity of the product.
Image 3. Refer to insert symbol indicates that the directions of use, warnings and/or list of ingredients can be found in the immediate vicinity of the product.
Eeva-Mari Karine, M.Sc.Eng.
Expert (cosmetics)
The Finnish Cosmetic and Hygiene Industry Association
Ritva Kurimo, M.Sc. (chemist)
TMI Ritva Kurimo